Files
Submission Date
Fall 2023
Abstract
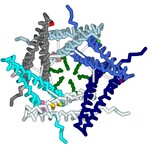
A type III secretion system (T3SS) is utilized by a variety of bacteria to inject toxins into host cells to cause disease, including Yersinia pestis, the bacterium that causes the bubonic plague. The Y. pestis T3SS needle subunit is a polymer made up of YscF proteins. Calcium is thought to play a role in regulation of toxin secretion by the YscF needle. High levels of Ca2+ binding inhibit secretion while low levels of Ca2+ binding cause secretion to be stimulated. According to a study done in 2005 by Torruellas et. al, different mutations of YscF can interfere with secretion regulation, most likely due to altered calcium interactions with the needle. Some details regarding which mutations lead to constitutive secretion (CS), no secretion (NS), or regulated secretion (RS) of the Yersinia outer protein (Yop) toxin are known. The goal of our research is to understand how the mutations present in previous wet lab experiments affect calcium binding to the YscF hexomer subunit. To understand if and how calcium interacts with the YscF needle to inhibit Yop secretion, an accurate model representation of the needle-calcium interactions is required. To model this phenomenon, the interactive accelerated protein prediction tool, CollabFold, was used to create probable hexamer subunits of the needle with the mutations from the previous experiment (Torruellas et. al, 2005). MIB2, a binding prediction modeling server, was used to predict binding with Ca2+, Fe2+, Fe3+, Zn2+, Cu2+, Mg2+. Alternative divalent cations were used to compare to Ca2+ binding to the hexamers. Ca2+ bound to the wild type (WT) hexamer at 12D, 15D, 16L; 28D, 29D; 36D, 37A with an average binding potential of 2.4. The binding sites contain aspartic acids that lead to CS when mutated to alanine, suggesting those binding sites play a role in calcium regulation of the needle. Zn2+ and Fe2+ bound to the WT hexamer at 56, 60 with an average binding potential of 4.0. Zn2+ and Fe2+ bound with higher binding potentials to the WT mutant hexamers on average compared to Ca2+. The hexamer structures of the different mutations were compared to look for structural differences between the mutations. Arginine 73 creates a ring in the center of the WT hexamer. The arginines at position 73 are further apart compared to the WT hexamer in the double mutant (D28A, D46A), I13A, and D17A CS hexamer mutations. The arginines at position 73 were obstructing the center passage in the N31A and D77A NS hexamer mutation. The arginine ring structural comparison between hexamers suggests that the R73 ring may play a role in regulating Yop secretion by obstructing the central needle passage. A 3-d printed model was created of a possible hexamer of the YscF Y. pestis needle subunit with the residue mutations causing constitutive secretion of Yop, and corresponding mutations indicating no Yop secretion, each highlighted in designated colors. The mutations indicating constitutive secretion are I13A, D17A, D28A, D46A. The mutations leading to no secretion are N31A, V34A, D77A, D77C, I82A, I82C. The results determined what mutations lead to CS, NS, or RS of the Yop toxin in the presence or absence of calcium.
Torruellas, J., Jackson, M., Pennock, J., Plano, G. (2005). The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Molecular Microbiology, Vol 57(6), 1719-1733, https://doi.org/10.1111/j.1365-2958.2005.04790.x
Recommended Citation
Olszewski, Kaya; Prasanna, Priyanka; Movva, Chetana; Sikora, Arthur; Schmitt Lavin, Emily; and Torruellas Garcia, Julie, "Modeling Calcium Binding to Yersinia pestis Type III Secretion Needle" (2023). Protein Modeling Reports. 16.
https://nsuworks.nova.edu/protein_modeling_reports/16
Poster Presentation
ModelingDivalentCationBindingtoY.PestisTypeIIISecretionHexamer.pdf (877 kB)
Modeling Divalent Cation Binding to Y. Pestis PowerPoint
JMOL Commands.pdf (103 kB)
Annotated Jmol Script to create Y. Pestis Hexamer model