Files
Submission Date
Fall 2023
Abstract
Cytochrome (CYP) enzymes are a superfamily of monooxygenase hemoprotein enzymes that are found throughout the body but are heavily concentrated in the endoplasmic reticulum and mitochondria of liver cells. These enzymes catalyze reactions that modify a wide range of substrates into more hydrophilic and, therefore, more readily excreted forms. Cytochrome enzymes are heavily involved in the detoxification process of many medically relevant drugs. As such, the inhibition and activation of these enzymes can substantially alter the effective bioavailability of medications and can introduce additional variables or modifiable variables into a pharmacological-based treatment.
Cytochrome p450 3A4 (CYP3A4) is one of the most abundant cytochrome enzymes and can target a wide range of substrates, including many medically relevant drugs. Inhibition of CYP3A4 can increase the bioavailability and duration of the availability of a medication in the bloodstream. This makes the factors associated with the inhibition and activation of CYP3A4 of great medical interest and importance. CYP3A4 has been noted to be inhibited by naringin, a flavanone found in grapefruits and other citrus fruits. However, the characteristics of naringin binding to CYP3A4 are unknown.
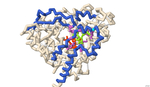
The residues at which naringin binds to CYP3A4 were identified using 3-D protein modeling and computerized molecular docking simulations. The PDB file for the CYP3A4 enzyme (8DYC) was obtained from the RCSB Protein Data Bank and was manipulated to remove the substrates, leaving only the prosthetic heme group. The binding between CYP3A4 and different inhibitors was studied in the literature to identify specific features of the CYP3A4 protein. The residues that form the opening of the protein cleft, the area where the substrates bind and the residues that form the active site of the enzyme were noted for reference for the docked position of naringin. PyRx was used to conduct molecular docking simulations to predict the location of naringin binding in the CYP3A4 structure. However, due to computational limitations, the docking did not predict interactions with the HEME group. Rather the final docked structure of naringin to the CYP3A4 enzyme was produced using the coordinates of the docked naringin and the HEME-containing structure of CYP3A4. The Contacts/Clashes functionality in USCF Chimera was used to identify the specific residues on the surface of the protein and these residues were compared to those identified through the literature consulted.
The residues of CYP3A4 that are involved in binding naringin that were identified through this process were: Glu 374, Arg 372, Arg 106, Arg 105, Ala 370, Phe 215, Arg 212, Phe 304, Leu 482, Ser 119, Ile 223, Thr 224. Of these, Arg 372 and Thr 224 were also noted in the literature to be involved in forming the opening of the cavity in the protein. The residues Glu 374, Arg 106, Arg 105, Ala 370, Phe 215, Arg 212, Phe 304, and Ser 119 were also noted in the literature and are likely involved in forming the active site. The residues Ile 223 and Leu 482 were not noted in the works consulted and may represent additional or potentially novel residues that may be involved in the inhibition of CYP3A4. Overall, the results of the molecular docking suggest that naringin inhibits CYP3A4 by binding and blocking both the opening of the cleft within which the substrate binds and binding to residues in the active site of CYP3A4.
Recommended Citation
Shah, Parth; Patel, Vraj; Ananthula, Sanjana; Schmitt Lavin, Emily; and Sikora, Arthur, "Identifying the Binding Residues on CYP3A4 to Naringin using Protein Modeling and Docking" (2023). Protein Modeling Reports. 15.
https://nsuworks.nova.edu/protein_modeling_reports/15
Annotated JMol Script
Poster .pdf (828 kB)
Poster
Presentation.pptx (454 kB)
Presentation