Files
Submission Date
Fall 2023
Abstract
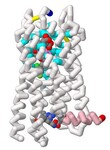
Underdiagnosed and under-treated, particularly in low- and middle-income countries, asthma has affected 262 million people globally. Current anti-asthmatic medications such as pranlukast inhibit cysteinyl leukotriene receptor 1 (CysLT1R), yet many patients do not respond to this drug. CysLT1R is associated with bronchoconstriction, inflammation, and mucus production in the airways of the lungs and bronchial tissues. When cysteinyl leukotrienes bind to CysLT1R, these effects are triggered contributing to the symptoms of asthma. The potential role of the related receptor CysLT2 in asthma remains poorly understood. To better understand this process, CysLT2R has been identified as a promising drug target for not only asthma but also other conditions such as brain injury and cancer. Students of the Honors Protein Modeling course at Nova Southeastern University modeled the interaction between a dual antagonist of CysLT1R and CysLT2R, KNW. A 3D model of KNW in complex with CysLT2R was based on PDB ID 6RZ6, modified using JMol, and 3D printed to showcase key interactions between drug and receptor. In this model of CysLT2R, we highlighted the ligand binding pocket, helix 8 (H8), and mutation residue interactions. The antagonist forms crucial interactions within the ligand-binding pocket (cyan). The N-linked carboxypropyl moiety forms salt bridges with Lys 37 and His 284 specific to CysLT2R (cpk). Mutating these residues to their CysLT1R counterparts decreases inhibition by antagonists. The key anchoring residue Tyr 119 interacts with benzoxazine, carboxylic groups, and amide linkers of the ligand (violet). The cleft opening residues include Leu 165, Val 208, and Tyr127 (light cyan). Unlike its counterpart, CysLT2R exhibits a wider cleft opening to the lipid membrane, enhancing ligand selectivity. Helix 8, a unique and flexible alpha-helix on the cytoplasmic side of the cell membrane, plays an important role in the regulation of G-protein activation and subsequent intracellular signaling cascades (pink). H8 conformation affects the binding site accessibility, signaling pathways, and receptor stability. Specifically, the salt bridge with Glu 310 stabilizes the junction between H8 and TM7 and the inactive state of the receptor when bound with the antagonist (cpk). Notably, the atopic asthma-associated mutation Met to Val in position 201 of CysLT2R results in a mildly impaired hypomorphic protein, with reduced ligand binding and inositol phosphate (IP) production (plum). Based on previously reported structure-activity relationship analysis, we developed a novel molecular inhibitor, Finlukast, aimed to have high affinity to both classes of receptors. Using SwissDock, we determined that this novel inhibitor molecule has high affinity binding to CysLT1 and CysLT2 receptors. Through the exploration and modeling of KNW, we gained further insight into the key structural interactions of dual antagonist KNW for similar receptor targets responsible for mediating the inflammation and bronchoconstrictive effects of cysteinyl leukotrienes.
Recommended Citation
James, Shalet; Jonnalagadda, Sreejani; Schmitt Lavin, Emily; and Sikora, Arthur, "Modeling Cysteinyl Leukotriene Receptor Antagonist KNW for Possible Optimized Asthma Treatment" (2023). Protein Modeling Reports. 12.
https://nsuworks.nova.edu/protein_modeling_reports/12