Files
Submission Date
Fall 2023
Abstract
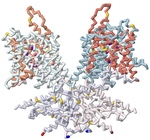
Prestin is one of the key motor proteins that has been identified in enabling auditory perception in mammals. By modulating its electromotility in response to changes in environmental voltage, prestin contracts and elongates in the plasma membrane of cochlear outer hair cells (OHCs). This allows different frequencies of sound to be processed quickly and precisely. Belonging to the SLC26A5 family of anion transporters, prestin is especially adept at binding anions in order to facilitate its oscillation through a series of unique conformations. Previous research has demonstrated that prestin is reversibly inhibited in the presence of salicylate. However, the broader mechanisms by which prestin senses and transduces voltage into cellular movement are not yet well understood. Previous studies have described the electromotility of prestin in terms of non-linear capacitance (NLC), wherein conformational changes in the protein are not linearly related to the voltage applied. High NLC is imperative for sound amplification in the cochlea, as this property enables OHCs’ selective response to different frequencies of incoming sound. 3D protein modeling was employed to better visualize the electromotility of this OHC protein by manipulating models of bottlenose dolphin (Tursiops truncatus) prestin available in the Protein Data Bank. Using the software PyMOL, Chain A of prestin in the inhibited state (7S9E) and Chain B of the compact, sensor-up state (7S8X) were spliced together into a novel merged model that depicts the fluctuation in the cross-sectional area of the transmembrane regions. This was possible because prestin is a protein homodimer whose peptide subunits could be swapped and replaced accordingly upon manipulation in the program. Key elements of prestin’s topology were then highlighted on this nascent model to emphasize the locations of the 14 gate and core transmembrane (TM) helices, the site of anion binding, and the cytosolic STAS domain. The colors corresponding with these regions include salmon, light blue, dark violet, and lavender, respectively. Helices TM3 and TM10 play a pivotal role in facilitating the movement of prestin’s dimers when bound to specific ligands. Likewise, the pocket formed by residues Gln97, Phe101, Phe137, Leu397, Ser398, and Arg399 has been identified as the anion binding site in the homodimer. The binding stability of ligands is further enhanced via additional noncovalent forces present in the active site, including pi stacking between salicylate and Phe137, and Ser393’s participation in hydrogen bonding. Arg399, the only positively charged residue in the cavity, is known to rotate up and down while the protein is moving, and neutralization of this key residue has also been found to eliminate prestin’s NLC entirely in vitro. Other residues of note that have been described by researchers include a series of 13 amino acid replacements (depicted in gold) that appear to be shared by several echolocating mammals, indicating convergent evolution between bats, whales, and dolphins.
Recommended Citation
Colon, Marqus; Hussain, Syed; Reside, Russel Robert; Schmitt-Lavin, Emily; and Sikora, Arthur, "Using 3D Modeling to Describe the Electromotility of the Outer Hair Cell Protein Prestin, and its Role in Sound Perception Among Mammals" (2023). Protein Modeling Reports. 14.
https://nsuworks.nova.edu/protein_modeling_reports/14